
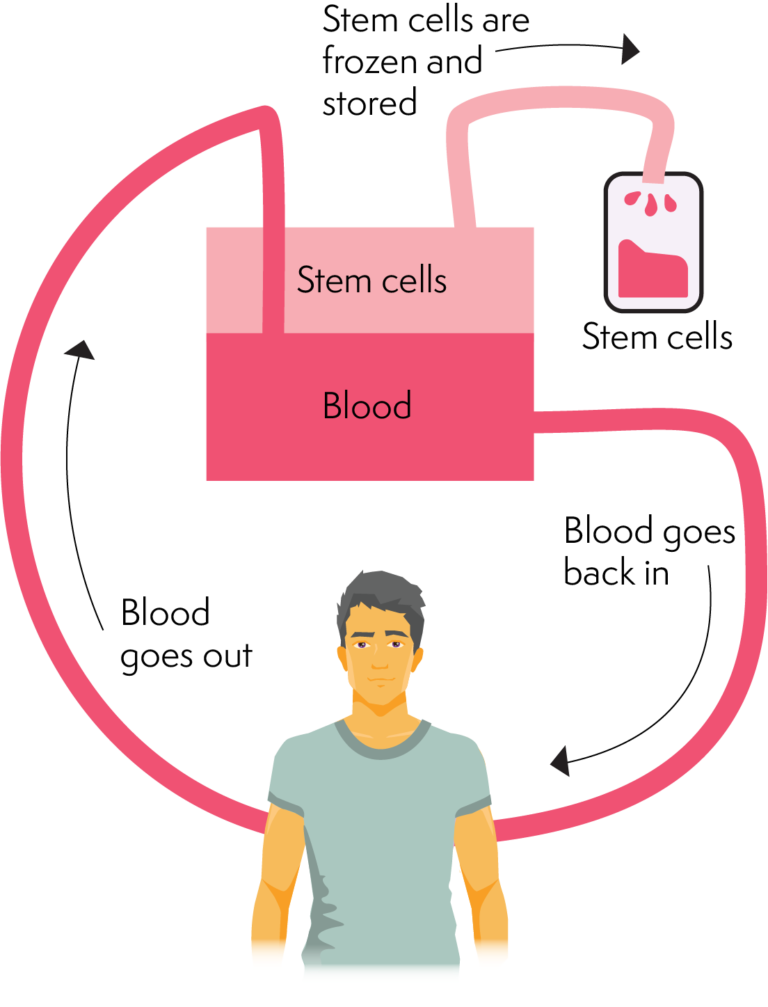
Non-Hodgkin's lymphoma (NHL) is an extremely heterogeneous group of lymphoid malignancies whose diversity relates to their epidemiology, natural history, morphology, immunology, cytogenetics and response to standard doses of chemotherapy. Malignant neoplasm of lymphoid, hematopoietic and related tissue ICD-10 codes covered if selection criteria are met: HCPCS codes covered if selection criteria are met:īone marrow or blood-derived stem-cells (peripheral or umbilical), allogeneic or autologous, harvesting, transplantation, and related complications including pheresis and cell preparation/storage marrow ablative therapy drugs, supplies, hospitalization with outpatient follow-up medical/surgical, diagnostic, emergency, and rehabilitative services and the number of days of pre-and post-transplant care in the global definition Histocompatibility/Blood Typing/Identity/Microsatellite Hematopoietic progenitor cell (HPC) allogeneic transplantation per donorīone Marrow or Stem Cell Services/Procedures Codes requiring a 7th character are represented by "+":ĬPT codes covered if selection criteria are met:īlood-derived hematopoietic progenitor cell harvesting for transplantation, per collection allogenicīone marrow harvesting for transplantation Information in the below has been added for clarification purposes.
#Autologous stem cell transplant icd 10 code
Table: CPT Codes / HCPCS Codes / ICD-10 Codes Code
Note: Aetna considers non-myeloablative allogeneic hematopoietic cell transplantation medically necessary ("mini-transplant", reduced intensity conditioning transplant) for the treatment of persons with relapsed NHL (including persons who have relapsed after ABMT) or primary refractory (see note below) NHL (low-grade, intermediate-grade, and high-grade) when they are eligible for conventional allografting or a reduced intensity regimen is preferred by the transplant center.Īetna considers tandem autologous hematopoietic cell transplantion (auto-auto) or tandem autologous hematopoietic cell transplantation followed by allogenic hematopoietic cell transplantation (auto-allo) experimental and investigational for NHL due to a lack of adequate evidence in the peer-reviewed published medical literature of their safety and effectiveness.



Footnotes for autologous hematopoietic cell transplantation* Note: Upon clinical review, Aetna may also consider autologous hematopoietic cell transplantation medically necessary for persons in first clinical remission with lymphoblastic NHL, Burkitt’s lymphoma, mediastinal B-cell lymphoma, mantle cell lymphoma, high-risk diffuse large B-cell lymphoma and other NHLs that are associated with poor prognosis andĮvidence of chemotherapy responsive (see note below) disease. In the absence of a protocol, Aetna considers autologous hematopoietic cell transplantation medically necessary for the treatment of NHL when all of the following selection criteria are met: Autologous Hematopoietic Cell TransplantationĪetna considers autologous hematopoietic cell transplantation for the treatment of persons with relapsed or primary refractory (see "Note" below) non-Hodgkin's lymphoma (NHL) medically necessary if the person meets the transplanting institution's protocol eligibility criteria.This Clinical Policy Bulletin addresses hematopoietic cell transplantation for non-hodgkin's lymphoma. Number: 0494 Table Of Contents Policy Applicable CPT / HCPCS / ICD-10 Codes Background References


 0 kommentar(er)
0 kommentar(er)
